Digitalization is a key factor and a prerequisite for the use of artificial intelligence in the pharmaceutical industry. Digitization allows the collection and analysis of large amounts of data, being an essential requirement for the development of precise and effective Artificial Intelligence models.

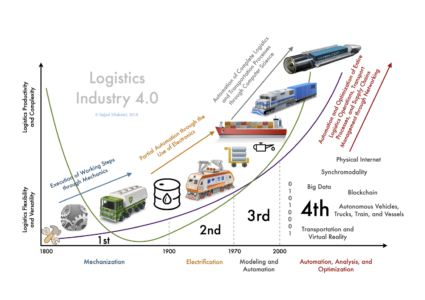
The fourth industrial revolution created the term “Industry 4.0”, characterized by the integration of advanced technologies such as Artificial Intelligence (AI), the Internet of Things (IoT), robotics or the automation of industrial processes. This integration also takes place in the pharmaceutical industry, and that is why the term Pharma 4.0 arises.
To be called a Pharma 4.0 business does not only refer to a name change, since it has a significant impact on the improvement of efficiency and quality of production processes, safety and effectiveness in the application of medicines, as well as the customer experience.
However, an essential prerequisite for the implementation of these technologies is digitalization. Thus, to achieve the benefits of “4.0 principles”, organizations must develop digital maturity, which means a certain level of development and advancement that allows them to use and take advantage of technologies efficiently and effectively.
How to achieve digital maturity?
Achieving the necessary level of maturity through digital transformation can be a very complex and even discouraging process due to the difficulty to implement the first steps. In fact, the level of maturity can be evaluated based on the measurement of different aspects such as technological infrastructure, organizational culture, employee recruitment, adoption of advanced technologies or process automation and systems integration.
Taking into account that each organization is a unique ecosystem, at least 3 basic steps can be established to achieve this digital maturity:
1.- Connect everything.
Connect as many elements as possible, including processes, instruments, products and people, ensuring that the data is accurate and reliable, so it should come mainly from clinical trials, electronic medical records, scientific literature, medical device data, among others. It is also needed to use advanced information management systems (AIMS) as they allow you to easily manage and control the information generated (samples, instruments, analysis of results and quality, etc.).
Automatically connected data will offer the following benefits:
- Mitigation of transcription errors and improvement of data quality.
- Freeing up time for staff, allowing them to dedicate this time to other tasks of greater value.
- Improved sample management for complete traceability.
- Real-time data management, access and reporting.

2.-End-to-end automated workflows.
Once data capabilities are connected, workflow automation solutions must be sought to accelerate and boost productivity. Therefore, process automation generates:
- Improved reproducibility to obtain reliable results.
- Reduction of manual handling errors and maximization of process performance. (By eliminating the need for repetitive and manual tasks, efficiency is improved and both the time and costs associated with the processes are reduced.)
- Increased collaboration and communication, encouraging the exchange of knowledge, joint decision-making and the identification of improvement opportunities.
- Guarantee of agility and flexibility, promoting rapid responses to market demands, changing regulations and patient needs.
- Continuous improvement and learning. Facilitating the identification of areas for improvement, implementation of changes and evaluation of impact in a systematic way, also adopting a mentality of constant learning.
As with valuable data collection, an integrated software environment combines data connectivity with workflow automation, promoting the linking of processes for the complete end-to-end workflow.
3.-Implementation of AI-based techniques.
Once data and workflow management is automated, there is a basis that allows data to be used efficiently and effectively thanks to Artificial Intelligence. There is a wide variety of applications that convert the collected data into valuable information for improving processes and decision making. This is why Artificial Intelligence allows:
- Patient care: personalization of patient care by identifying disease patterns based on their prevention.
- Quality control: detection of patterns and anomalies both in machinery (preventive maintenance) and in compounds that indicate in advance problems in the production of medicines.
- Logistics and distribution: optimization of the supply chain, from production processes to the marketing of products, reducing costs and improving efficiency.
- Clinical trials: identification of patients suitable for trials, dose selection and analysis of clinical results.
- Drug discovery and design: identification of promising new drug compounds and optimization of them to improve their effectiveness, safety and even bioavailability.
However, these applications will only be useful if they are implemented correctly, so it is vital to identify and define the problems that AI can help solve since its applications are most effective especially when they focus on a specific challenge.
The identification and definition of a specific problem, as well as the creation of the appropriate algorithms that translate the data into solutions for decision-making, is not trivial, as it requires great experience and care in reading the needs of companies.

In conclusion, AI is having a profound impact on the pharmaceutical industry, enabling advances in key areas such as drug research and production. As technology continues to evolve, it is likely that Artificial intelligence continue to transform the way medicines are developed and distributed around the world. Reason why it is necessary to continue working to ensure its ethical and safe use.
baobab soluciones is the answer if what you are looking for is to accelerate innovation in your company through AI solutions,with the use of the most advanced techniques such as Operational Research! Contact us now and start the path towards Pharma 4.0, enjoy the benefits that advanced analytics can provide your business!
Click on the link to learn more about our solutions.
References
- Arden, N. S., Fisher, A., Tyner, K. M., Yu, L. X., Lee, S. L., & Kopcha, M. (2021). Industry 4.0 for pharmaceutical manufacturing: Preparing for the smart factories of the future. International Journal of Pharmaceutics, 602, 120554. https://doi.org/10.1016/j.ijpharm.2021.120554
- Marketing Team. (2023, 23 enero). Digital Maturity in Pharma: How digital is the pharmaceutical industry? Doxee. https://www.doxee.com/blog/digital-disruption/digital-maturity-in-pharma-industry/
- Pharma 4.0: How connectivity, automation and advanced analytics accelerate innovation. (2023, 3 mayo). Scientific Computing World. https://www.scientific-computing.com/viewpoint/pharma-40-how-connectivity-automation-and-advanced-analytics-accelerate-innovation